

Which is your favorite molecule? Perhaps the double helix, because it unites everything that defines us? Or the caffeine, because it reliably gets you going every morning? Or is it the 1,4-diphenyl something, because the synthesis and isolation of exactly this substance was the breakthrough in your doctoral thesis? No matter which molecule (or compound) is your favorite, complex or mundane, known or not, let us know. In the anniversary year 2017, we are introducing your favorite molecule here. In addition to an illustration, structural formula, space-filling model, etc. of the molecule, you should provide a brief explanation. This does not have to be strictly scientific, but can also be original, philosophical, in poetry, etc. Whatever you can think of about your favorite molecule or compound, drop us a line. At the end of the anniversary year, we will raffle ten Amazon shopping vouchers worth EUR 20 each among all senders. Everything comes to an end once, including our "Favorite Molecule" campaign in the 2017 anniversary year.
What began with the sweet switches, we end with the 2,4,6-trinitrotoluene. You have sent us a total of 61 molecules (and some elements) for our anniversary year. Her favorite connections were small or large, simple or complex, but always beautiful. We thank all participants for their submissions. The winners of the shopping vouchers have now been determined and notified.
Every week we present one of our members' favorite molecules. You can see the individual molecules by clicking on the arrow to the right of the name.

My favorite molecule is 2,4,6-trinitrotoluene, or TNT for short. On the one hand, this is due to its simple, symmetrical beauty, and on the other hand, because I worked intensively on it during my doctoral studies. Since then I have also been wearing a ring that is supposed to symbolize the molecule, but not completely chemically correct, as the goldsmith refused to depict the nitro groups correctly. In addition, TNT embodies energy for me and is at the same time a reminder of the responsibility of natural scientists that every helpful invention can have devastating effects in the wrong hands. Last but not least, there is also a connection to my second passion, music: TNT is one of the few molecules that has been set to music several times. Not only in the well-known AC-DC version, but also in 1925 by Fletcher Henderson and his orchestra. By the way, and this is where he came full circle, he had earned a bachelor's degree in chemistry before starting his Career as a band leader. Mario Kröger, Bruchsal
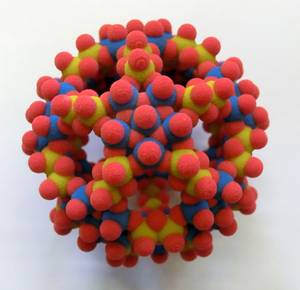
My favorite is the inorganic football molecule Mo132, which has occupied me with its dynamics in solution for years and always fascinates anew with its diversity. Erhard Haupt, Tornesch
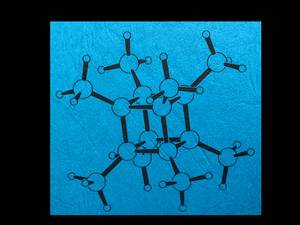
My favorite molecule is octamethylcubane, which I synthesized photochemically. I am fascinated by its high symmetry and aesthetics as well as the very simple 1H-NMR spectrum due to 24 equivalent protons. An attractive molecule with rough edges - and yet somehow spherical. Stefan Brand, Hirschberg-Leutershausen
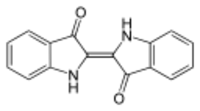
Indigo is one of the first natural dyes whose structure was clarified (A. von Baeyer, 1883) and whose technical synthesis was realized (Heumann, BASF, 1890). In the working group of my doctoral supervisor, Wolfgang Lüttke, University of Göttingen, the chromophoric stem system (urindigo) of this deep blue dye (longest wave band at 606 nm) was determined by quantum chemical calculations and experimental synthesis. Georg Becher, Oslo Graphics: Wikipedia Commons
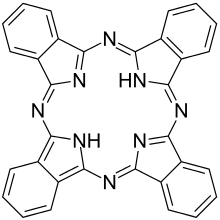
My favorite molecule is phthalocyanine (PcH2), discovered by RP Linstead in 1933, the structure of which was confirmed by X-ray structure analysis by JM Robertson in 1935. As a tetradentate ligand, it can form very stable complexes with numerous elements. Rudolf Taube was able to show in the 1960s that the phthalocyanines of the 3d elements can be reduced in several stages while maintaining their structure. As part of my diploma thesis in 1972, I was fortunate to investigate the reduction of aluminum (III) phthalocyanine chloride in analogy to the reduction described by Taube and to obtain the first reduction stage as AlPc ? 3THF in the form of shiny golden brown crystals, the Some time ago I handed it over to the historical dye collection of the TU Dresden for safekeeping in an airtight, melted substance tube. Before that, I had already succeeded in synthesizing the soluble vanadyl phthalocyanine on behalf of Carl Zeiss Jena, which served as a switching substance for giant pulse lasers. It is known that phthalocyanines found widespread use as dyes and pigments soon after their discovery. They were later used as photoconductors in laser printers or as electrode material in fuel cells. Phthalocyanine derivatives have recently received particularly strong attention due to their use in photodynamic tumor therapy.
Vladimir Reshetilovsky, Radebeul
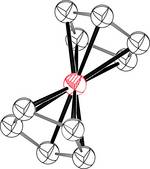
Ferrocene was the cornerstone of the discovery of organometallic chemistry. It is through this simple molecule that the concept of a carbon-metal bond has been discovered, without which we would have no understanding of homogeneous catalysis. The structure elucidation of this molecule was so groundbreaking that it was honored with a Nobel Prize. In addition to its structure, this molecule is also highly reversibly oxidizable, making it THE redox standard in organic solvents that is still in daily use today. Christopher Hassenrück, Constance
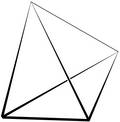
My favorite molecule is tricyclo [1.1.0.02,4] butane, better known under the short name tetrahedrane. A highly symmetrical, as well as inaccessible molecule. At first glance, however, this cannot be seen, because if you ask beginners in organic chemistry whether you can make this connection, in most cases they will say 'Yes!'. Only later do they realize the impossibility. Andreas Pingel Keuth, Düren

My favorite element has 12 electrons,
this is as clear in the PSE as it is in cement.
It fulfills many functions in the body.
For example, everyone runs even more disturbed in the marathon.
Even in royal chess
all grandmasters play very weakly without you.
Magnesium is my favorite element
and therefore I don't only like it in Advent.
Benjamin Ehrlich, Sigmaringen
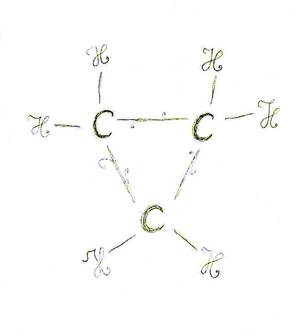
Cyclopropane is small, but very exciting. Above all, however, my neighbor at the bank drew a wonderfully playful picture of it in my poetry album at the beginning of our chemistry training.
Maren Bulmahn, Frankfurt
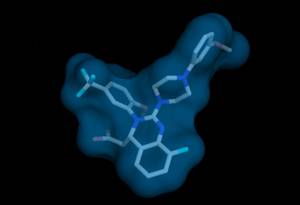
This substance called Letermovir ((4S) -8-Fluoro-3,4-dihydro-2- [4- (3-methoxyphenyl) -1-piperazinyl] -3- [2-methoxy-5- (trifluoromethyl) phenyl] - 4-quinazolineacetic acid) was developed for the prophylaxis and treatment of cytomegalovirus infections in immunocomprimized patients like transplant recipients. A recent phase III trial in hematopoetic stem cell recipients demonstrated not only strong suppression of the virus by the drug, but most importantly, fewer deaths among those patients who were treated with this novel, first-in-class molecule.
Helga Rübsamen-Schaeff, Wuppertal
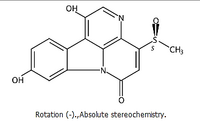
Curtisin is my personal favorite molecule because it 'accompanied' me during my thesis and for a long time afterwards. Due to a small amount, I was able to derive partial structures at the time, but unfortunately I could not predict the complete structure.
Only after further work could the structure of the dye be fully elucidated. This connection was unusual for boletus at the time and exemplarily reflects the fascination of natural product chemistry. Furthermore, the structure elucidation by Curtisin also shows that it took the work of several natural scientists to get a complete picture.
Martin Bröckelmann, Rüsselsheim

My favorite molecule is bisphenol. I particularly like it in connection with a composite bicycle that rides out of a benzene ring of bisphenol, like on this book cover. Dr. Egbert Brandau, Karlstein
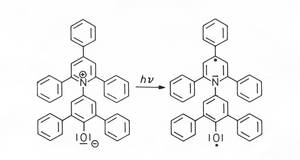
This negatively solvatochromic betaine dye shows an extreme dependence of the long wavy Vis absorption band on the polarity of the solvent: the solution colors are red in methanol, violet in ethanol, blue in i-amyl alcohol, green in acetone and yellow in anisole. With increasing solvent polarity, the intramolecular charge-transfer absorption band is shifted hypsochromically by up to about 360 nm, which has been used to establish a useful empirical scale of solvent polarity: the ET (30) or ETN scale. The emotional connection with this dye stems from the fact that I succeeded in producing the first ?world supply? of this dye in 1962 as part of my dissertation. It is now also available in stores and anyone can experiment with it.
Christian Reichardt, Marburg
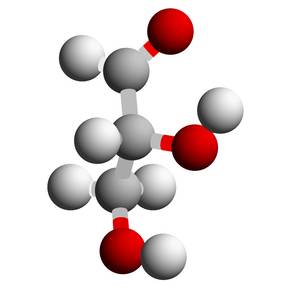
D-glyceraldehyde is a member of the group of the simplest carbohydrates, the trioses, which have a chiral center in the middle of the molecule. It is part of what is probably the earliest probiotic metabolism and also part of all metabolic processes in living cells. In addition, it is in constant equilibrium with the symmetrical dihydroxy acetone and L-glyceraldehyde in the form of a keto-enol tautomerism. D- and L-glyceraldehyde are diastereomers, so they are an image and a mirror image. Nevertheless, only D-glyceraldehyde plays a role in the metabolism of living organisms on this planet.
This in turn leads to one of the most exciting and so far unsolved questions that occupy the natural sciences: Why do only D-sugars and L-amino acids play a role in metabolism in living cells, or: How did the spontaneous symmetry break (homochirality) come about? Probably a decisive step can be taken to distinguish living from non-living matter if one can find out how this particular spontaneous symmetry break came about.
Peter Friedel, Dresden
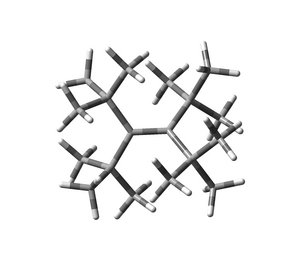
My favorite molecule is tetra-tert-butylethylene. Basically a simple molecule.
Two generations have dealt with synthesis, but have not found a way into the molecule. Many properties have been calculated, including a possible synthetic route, but the connection has not yet been established.
Dieter Lenoir, Munich
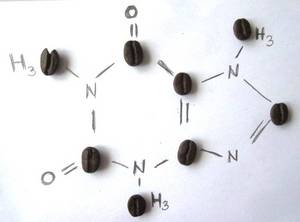
My favorite molecule is caffeine. Without our completely legal stimulant, many of us would be lost day and night! Without caffeine, there would be far fewer creative ideas, no meaningful project meetings and no efficient night shifts at large research equipment. Caffeine helps bachelor and master theses, dissertations, third-party funding applications and reports to be completed on time and better.
What more could you want from a molecule?
Silke Merchel, Dresden
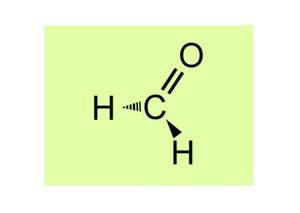
My personal favorite molecule is called formaldehyde. Internationally, this methanal can be easily assigned both because of its formula and because of its name, which sounds the same in many languages, and because of its authenticity, it has the highest social function, in particular because of its properties that encourage discussion. Based on the possibility to philosophize germanophon whether it is better or equivalent to formaldehyde, the communicative challenge increases because, depending on personal focus, it can be a short-lived biochemically vital body's own mediator or a classifiable cancer-promoting hazardous substance. Thanks to the activation of my laughing muscle proteins, this molecule makes me in a good mood and, thanks to sufficient concentration in the air I breathe, makes me cry as a warning when processing the Solutio Formaldehyde. It's a real love affair with all its ups and downs.
Erich Leitner, Bruck / Austria
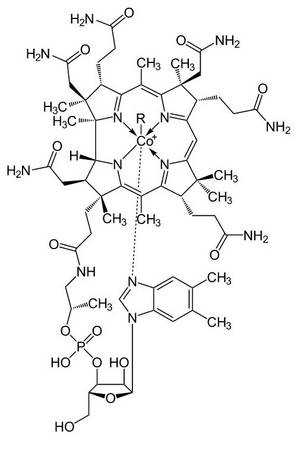
One of my favorite molecules is vitamin B12 because it is one of the few organometallic compounds in nature and there catalyzes unusual rearrangements. For me it connects many areas of organic, organometallic, biochemical and pharmaceutical chemistry. In addition, the molecule went down as a milestone in the history of organic chemistry. In an unprecedented cooperation in the groups of Prof. A. Eschenmoser and Prof. RB Woodward, it was represented and characterized in a fully synthetic way for the first time in the 1970s.
Friedrich Kroll, Dülmen

My favorite element is silicon. Firstly, because the color and properties are very special. I find the gray-black color of the semi-metal, which has a metallic, but often very chic bronze to bluish sheen, particularly beautiful and fascinating. In addition, silicon is very important as a semi-metal and especially a semiconductor. Today's electronics industry would be unthinkable without the silicon used in processors and transistors. Silicon is not only the second most abundant element in the earth's shell because of its oxides, but it is also widespread throughout the world and can be found in various electronics in each of us.
Tino Beste, Münster
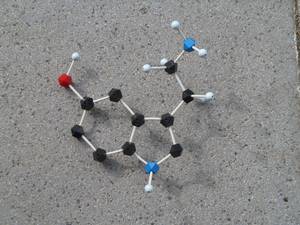
Serotonin, the happiness hormone, is involved in the regulation of emotions, learning processes and endocrine functions.
Anke Dreyer, Aachen
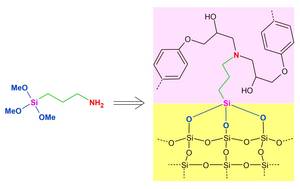
(3-Aminopropyl) trimethoxysilane is a member of a whole class of reactive silicon compounds, the organofunctional silanes. These silanes are links between organic and inorganic substances and materials: With their hydrolyzable groups, they bind to inorganic surfaces and their organic residues and functional groups determine the physicochemical properties and can be reactively bound to organic materials.
These organofunctional silanes have been with me throughout my entire academic and professional career: from my main studies to my doctorate, in my industrial work and now in my position as a university professor. They are ?interdisciplinary? molecules and prove what Friedrich Wöhler showed in his famous experiment in 1828: There is no separation of inorganic and organic chemistry, but the principles of chemistry are equally valid for all substances and materials. Dennis Troegel, Nuremberg
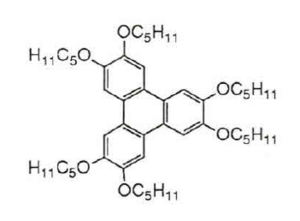
H5T, as we called it, more precisely 2,3,6,7,10,11-Hexakis (pentyloxy) triphenylene, is a discotic, i.e. a disk-shaped liquid crystal. The aim was to develop a better photoconductor from this electron donor with the help of electron acceptors.
One day a film team came to our university in Mainz to apply for chemistry studies, and so H5T was drawn on the blackboard by an actor-professor. I'm proud of my prompted sentence "And next week I'll show you how to attach hexapentyloxytriphenylene to a polymer chain in order to improve the material properties".
Holger Bengs, Frankfurt
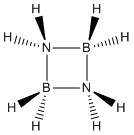
Let's talk about small rings. Do you know the smallest genuinely inorganic ring? Here it is: Cyclodiborazane (BH 2 NH 2 ) 2. It really does exist, the isosteric cousin of cyclobutadiene, as a mimosa-like solid: JACS 88 (1966) 4396.
Karl Wilhelm Böddeker, Hamburg
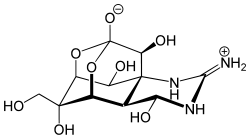
My favorite molecule is tetrodotoxin (TTX), the deadly poison (= neurotoxin) of the Chinese / Japanese puffer fish "Fugu". Only selected chefs with a special license and years of experience are allowed to prepare and offer this fish in special restaurants. I have had the privilege of being invited to a fugu meal (a ritual) once in China and twice in Japan. TTX is an alkaloid from the imidazoline and pyrimidine groups. It blocks tension-charged sodium channels, which leads to muscle paralysis. It's one of the most powerful poisons ever - the lethal dose is 10 micrograms per kilogram of body weight.
Hans Joachim Gross, Würzburg
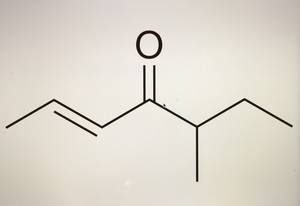
5-Methyl-2E-hepten-4-one (Filbertone) is one of the key flavorings in roasted hazelnuts (Filberts). In the form of the pure component, its smell is described as "nutty, hazelnut, metallic, buttery, soft". The compound has one of the lowest odor thresholds known to the human sense of smell and is already perceived in the ppt range. This and many other molecules from the fascinating world of smell and taste have accompanied me throughout my Career . Of the corresponding isomers, (+) - 5 (S) -methyl-2E-hepten-4-one is a particularly odor-active one. In addition to the natural occurrence in hazelnuts, the compound has also been used commercially as a flavoring substance for many years. Matthias A. Güntert, Ridgewood, USA
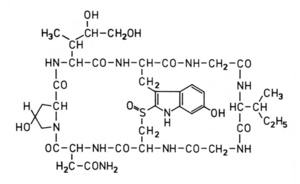
Almost fifty years of intensive research on Amanitin creates an emotional bond with this molecule. The poison of the green leaf mushroom still claims the deaths of mushroom pickers every year - despite the antidote silibin available today. Due to the strong and specific inhibition of the transcription enzyme RNA polymerase II, Amanitin has also become a valued tool in molecular biology. And in Medicine could Amanitin covalently bound to tumor-specific antibodies, acquire in the near future as an anticancer agent meaning.
Heinz Faulstich, Heidelberg
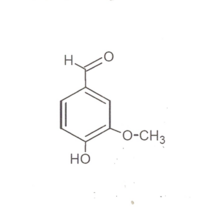
It was a groundbreaking success in science that in the 19th century the Hofmann students Wilhelm Haarmann and Ferdinand Tiemann succeeded in elucidating the structural formula of the natural substance vanillin and discovering the chemical synthesis based on spruce juice:
.
150 years ago in Berlin, when Hofmann became president,the formula vanillin was found, and Haarmann was the assistant.
I looked into the matter, thoroughly researched the event,
then caught the doctoral cap, wrote everything down: "Rich Man The Lord of Scent".
Björn Bernhard Kuhse, Halle / Westf.
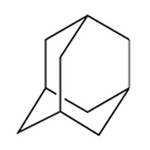
I did my FH diploma thesis in 1981 at the University of Tübingen in the department of Organic Chemistry. A doctoral student in this working group is engaged in NMR studies on various methylated and phenylated adamantane derivatives. That was when I first heard of adamantane. I was impressed that he said in Swabian that the adamantane structure was like small "spheres" and that it was therefore very volatile. He must never forget to close the pointed flasks, otherwise the painstakingly synthesized compound would be gone. Even today I particularly like this "spherical" molecular structure of adamantane.
Andreas Weber, Wiesbaden
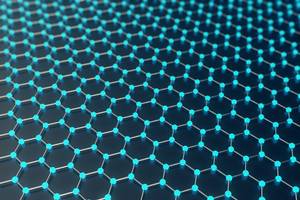
As a polymer chemist, I am fascinated by simple units with multiple effects. Graphs offer a simple basis like with LEGO bricks. It is stable and still modifiable. There are numerous applications and areas of research. Graph is exciting. A giant molecule? A single crystal? Or both ?
Heinz Herzog, Herzogenrath
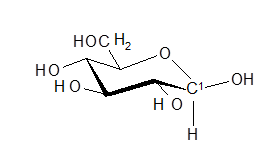
Glucose is a versatile, proven example of general aspects such as constitutional isomerism, enantiomerism, diastereomerism and reactivity of functional groups in the classroom. Some chemists do not like carbohydrates, including their flagship, because the structure and how to determine them are considered complicated.
Glucose is my preferred molecule because the question "How do I tell my student?" Can now be answered fairly easily using appropriate X-ray structure determinations, e.g. using ß-D-glucose, in which all 5 substituents are in the equatorial positions Here, too, my favorite molecule is an example of something more general, namely of various advances in the study of crystals.
Albrecht Mannschreck, Regensburg
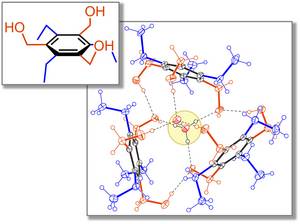
My favorite molecule is the tripodal compound 1,3,5-tris (hydroxymethyl) -2,4,6-triethylbenzene, because in the crystalline solid three of these molecules enclose an ion pair of H 3 O + and OH - ions and through the formation effectively stabilize hydrogen bonds. The preorganized hydroxyl groups take on the role of the water molecules of postulated, hydrated hydronium or hydroxide-ion complexes.
Manuel Stapf, Freiberg
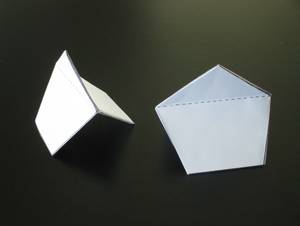
In the 1997/98 winter semester, I gave a lecture on ?Molecular models made of paper? at the University of Bern, although the department management did not find this topic enthusiastic. I had found suitable templates in various magazines, and I created my own ideas for some models. But then, after a very difficult year personally in 1997, it occurred to me on New Year's Eve 1998 how to make Norbornan C 7 H 12 using the classic origami technique - that is, from a square piece of paper, only with folds, but without cuts or glue could. In the morning I sat behind it and I was able to fold a norbornan straight away! It was like a promise that I would be able to face the new year. Veronika Meyer, St Gallen, Switzerland
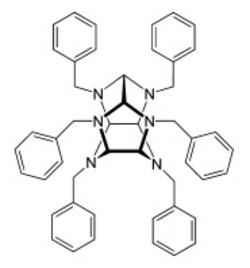
When producing the explosive Cl-20 (hexanitroisowurtzitane), the hexabenzyl derivative 2,4,6,8,10,12-hexabenzylhexaazaisowurtzitane - my favorite compound - is obtained in the first reaction step of the three-stage synthesis.
Why? Completely without any serious, scientific background - it looks like a "hunter" from the hunter program of the Hollywood film "Pacific Rim", or an "Evangelion" from the original "Neon Genesis Evangelion", a huge, hybrid, human-controlled machine that does it has to take on enormous, misanthropic opponents.
Kevin Hangleiter, Dornstadt
Phenothiazine was a loyal companion to me during my time as a graduate and doctoral student at the university. It is an organic compound that has an aromatic and a heterocyclic character, but only its electrochemical properties make it particularly interesting. We had suffered several challenging days of what felt like never-ending chromatography, as well as many wonderful moments, for example when syntheses and purification steps were successful or our articles were accepted for publication. Above all, phenothiazine taught me patience and tenacity, which I will draw on for a long time, both privately and in my professional life.
Adam Franz, Weinheim
1,7-substituted anthracenes have fascinated mankind since the dawn of science and have brought about numerous everyday blessings as a by-product of their research. At least they are visually extremely appealing, be it as a material with terrific luminosity, especially under UV light, and also as a structural formula on paper. They are unsurpassed as structuring agents and spacers, unfortunately many shy away from the sensitivity of the anthracene unit and switch to benzophenone units, for example (not bad either, but nothing beats the original).
Ralf Hauck, Basel, Switzerland
All people from West to Polonia
were hungry or they had pneumonia
Along came Haber and Bosch
gave air to almighty push
and a catalyst to produce ammonia.
Wolfgang Gerhartz, Zwingenberg
Fig: Wikipedia Commons (https://commons.wikimedia.org/wiki/File%3AAmmoniak.svg)
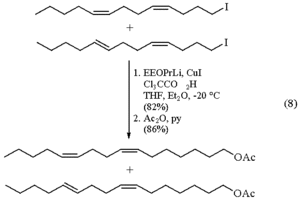
"Gossyplure", first described in Science in 1973, is a sex pheromone of the cotton moth Pectinophora gossypiella Saunders (Lep: Gelechiidae), a mixture of two isomer pairs, of (Z, Z) - and (Z, E) -7,11-hexadecadienyl acetate.
In July 1973 it was the most valuable natural substance that could be imagined in the fight against the cotton moth that was introduced to California and is an original example of non-toxic plant protection using the mating disorder method. The immediate assignment in the IPM in Californian cotton fields under swarms of mosquitoes was a sweaty, but worthwhile assignment.
Non-destructive spectroscopic investigations by IR, UV and NMR, as well as the controlled ozone depletion, which provided three fragments that could be characterized by gas chromatography, were sufficient to elucidate the structure of 0.3 mg, which had been painstakingly isolated. The EI-MS and the GC retention times confirmed the structure. The structural proof was carried out by means of total synthesis.
Hans E. Hummel, Giessen
Very nice symmetrical, just look, that's my dear melamine.
When it is combined with aldehyde, a resin of the highest quality is created.
Resistant to heat and fire and ultimately not even that expensive.
A foam can be made out of it and many other things.
Heinz Weber, Grünstadt
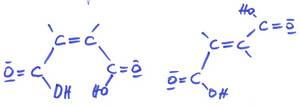
I really love this conjugated system in both editions with so incredibly different properties, and I actually mean the beauty of the molecular structure. Back then, I was working on esters that were previously unknown and doing my doctorate. Hans-Albrecht Meyer-Stoll, Moers
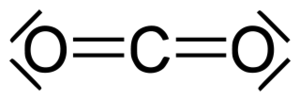
As can be seen, CO 2 is an extremely elegant molecule: its straightness (linearity), its robustness (stability) and its serenity (inertia) are captivating. CO 2 is therefore an omnipresent component of the atmosphere, the hydrosphere and the lithosphere. In the biosphere, CO 2 is an essential part of the global carbon cycle and therefore a basis of life on our planet. The extensive use of fossil carbon sources for energy generation by humans has brought CO 2 into disrepute as a climate killer through no fault of their own; it requires the rescue of honor through chemistry.
We are working on it: the use of CO 2 as an alternative carbon source is the subject of growing international research, industrial use is moving into the realm of what is possible and sensible.
Peter Orth, Cologne
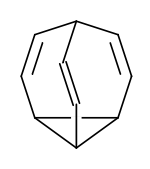
Bullvalen is my favorite molecule because at low temperature it has differently bonded hydrogen atoms, but at higher temperatures they are all in the same bond state.
Kurt Wendel, Ludwigshafen
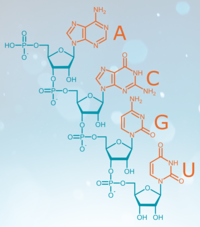
RNA is an incredibly versatile and fascinating molecule. Presumably, RNA is the first biomolecule to emerge and therefore the basis of all life. Nowadays, in addition to the catalytic functions, the medical potential of this substance, which has been neglected for a long time, is being researched at high pressure. Contrary to the prevailing doctrine to date, it turned out that the RNA itself is very stable and can be used medically as a messenger substance for building instructions for proteins. Cancer therapies, prophylactic vaccines and molecular therapies are currently being researched and developed. RNA thus achieves the incredible balancing act from the origin of all life to the most modern drug development.
Dr. Wolfgang Große, Tübingen
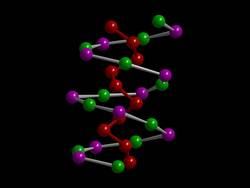
I would like to suggest the new type of semiconductor material published by Prof. Tom Nilges as a favorite molecule with its own mechanical properties and great potential for adaptation through substitution. I read it in an article by the Technical University of Munich and later attended a coffee talk by Prof. Nilges personally. It consists of the first inorganic double helix and should not be missing here.
Markus Pielmeier, Munich

"Eventually the whole thing dissolves into systems of formulas that are somehow related to one another, and there are only a few dozen people in the wide world who think the same thing about something as simple as water; everyone else talks about it in languages that are at home somewhere between today and a few thousand years ago. So it must be said that if a person just thinks a little, he ends up in a very messy company, so to speak! "
(Robert Musil, The Man Without Qualities, Book One, Part Two, Chapter 28)
Ulrich Sattler, Berlin
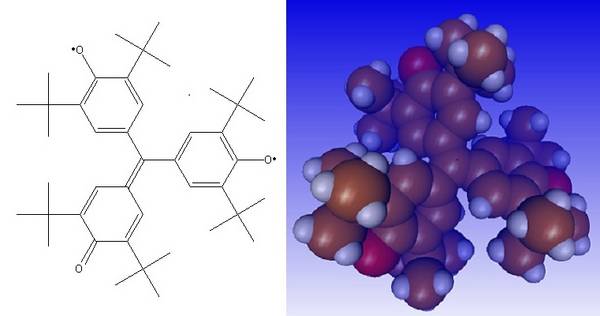
The stable Yangs biradical with a triplet basic state occupied me in my diploma thesis and afterwards for several years. It has threefold symmetry, possibly only in terms of time average, was examined according to all the rules of the art, focusing on magnetic resonance (EPR / ENDOR), without revealing any secrets. Its diamond precursor, called HTA ("hexa-tert-butylaurine"), was also formed from unexpected precursors (e.g. diethyl oxalate) in organometallic syntheses.
Burkhard Kirste, Berlin

Copper is my favorite element. It connects the topic of my doctorate with one of the finest liquors, Scottish malt whiskey, for whose production it is indispensable as the material of the still.
Horst Klassen, Erftstadt
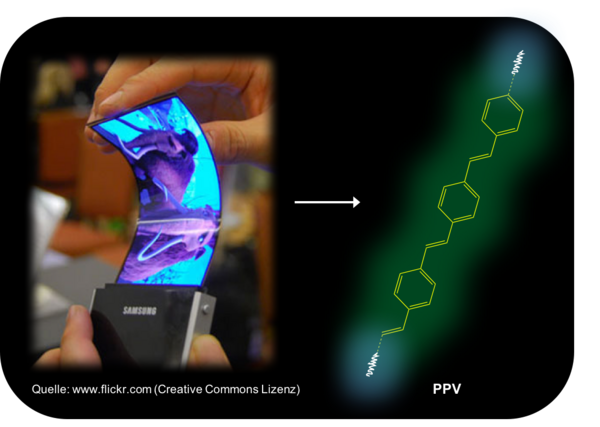
Semiconducting polymers such as poly (para-phenylene-vinylene) - PPV for short - form the active photo layers in applications such as flexible AMOLED displays (Active Matrix Organic LED) or organic solar cell films. This topic is a prime example of the further development of scientific concepts. Because PPV is not an electrical insulator like conventional plastics. During the synthesis of these high-tech polymers, a continuously conjugated double bond system is formed, which gives the polymer molecules the ability to transport charges and interact with visible light. A fine example of the structure-property concept of chemistry. My personal favorite is the Superyellow, a derivative of the PPV. With this polymer I was able to develop a low-cost OLED for school lessons and science communication in my doctoral thesis: the breakthrough in my scientific Career.
Amitabh Banerji, Cologne
Because there is little that holds together so tightly. Eliza Leusmann, Frankfurt aM
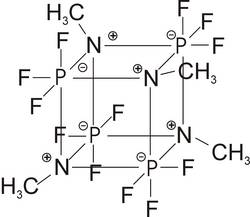
Dimer 2,2,2,4,4,4-hexafluoro-1,3-dimethyl-1,3,2,4-diazadiphosphetidine [(CH 3 NPF 3 ) 2 ] 2 or more simply according to the old nomenclature: tetrameric methyliminophosphoric acid trifluoride (CH 3 NPF 3 ) 4. One of the few purely non-metallic molecules with a cube-shaped basic structure in analogy to Kuban C 8 H 8. A compound with a quadruple zwitterionic structure, thermally and hydrolytically remarkably stable. In the regularity of the three-dimensional arrangement with high symmetry of the interpenetrating phosphorus and nitrogen tetrahedra, it is simply a ?beautiful? molecule.
Michael Charwath, Laxenburg, Austria
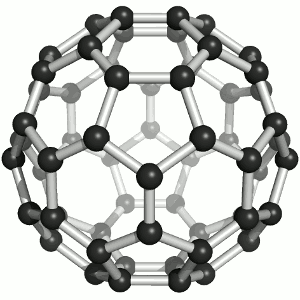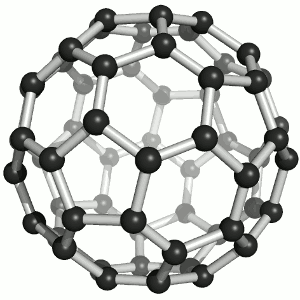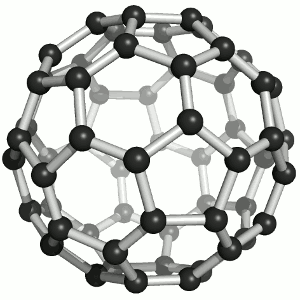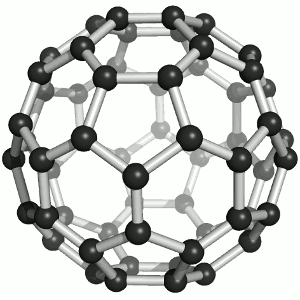
Two members have chosen the fullerene as their favorite molecule:
It offers fascinating beauty for laypeople and experts alike. A coincidental discovery story and characterization with a crowning Nobel Prize, perfect harmony / symmetry, the reference to a mass hobby (more men) and aspiration for a luxury Buckydiamond (more women) that is created under high pressure. It offers incredibly eclectic inspiration in virtually any potential research area. But the conclusion for me is that it can be created with practically any normal soot, for example on the Christmas candle.
I therefore hope that Buckminsterfulleren will inspire hosts of bright people to take up natural science and thus make the world rounder.
Jens Kremeskötter, Ludwigshafen
___________________________________
On the one hand, it impresses with its aesthetics: its geometry, its structural grace as well as the color of its solutions. On the other hand, for me it symbolizes a bridge to nanotechnology with numerous - at least conceivable - applications. Last but not least, after its 'renewed' discovery and structure elucidation at the MPI for Nuclear Physics in Heidelberg, I was one of the first chemists to work with it as part of my chemistry studies. :-)
Matthias Brunner, Heidelberg
Image: Sponk,Buckminsterfullerene animated, CC BY-SA 3.0 (https://commons.wikimedia.org/wiki/File:Buckminsterfullerene_animated.gif)
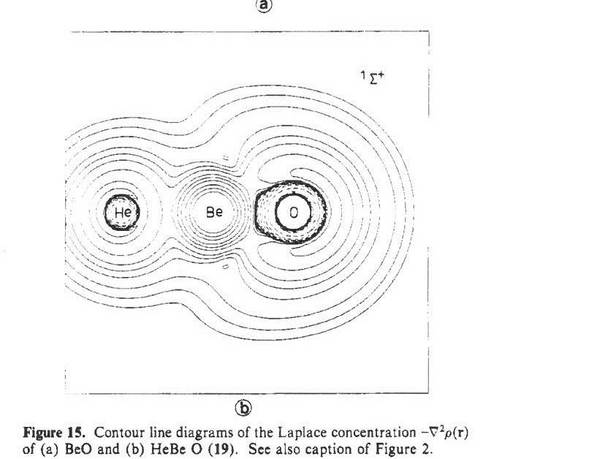
The first neutral molecule, quantum-chemically predicted in 1986, in which helium somewhat relativizes its nobility and forms a bond. Using state-of-the-art computing methods at the time, Gernot Frenking, Jack Collins and I were able to determine a He-Be binding energy of a few kcal / mol, so the molecule should in principle be observable in the gas phase. This prediction attracted some attention and made it to the New York Times (?On Elements That Want to Be Alone?) and the FAZ; the experimental confirmation is still pending (the heavier element homologues, on the other hand, have been identified experimentally).
Wolfram Koch, Frankfurt aM
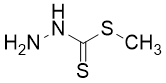
My favorite molecule is methylhydrazine carbodithioate. It is the starting molecule for my master?s thesis with a very interesting and diverse chemistry. Only my laboratory partners do not like the synthesis of the molecule, after all it stinks a lot.
Alexander Haseloer, Cologne

My favorite molecule is the fluorescent dye S-13 (RN 110590-84-6) because its development represented a breakthrough in the chemistry of soluble perylene fluorescent dyes and other derivatives. These are not only interesting as versatile optical functional materials, but are also aesthetically pleasing due to their beautiful variety of colors.
Heinz Langhals, Munich
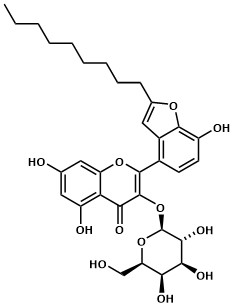
My favorite molecule: Houttuynoid B. I was able to fully synthesize this molecule for the first time in 2015 after years of research. I put a lot of time, both physical and intellectual, but just as much frustration into the total synthesis of this molecule - so it has had a more than lasting impact on my life.
Thomas Kerl, Cologne
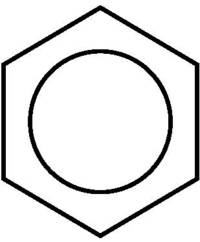
The regular hexagon of the benzene molecular skeleton is an icon of chemistry - scientifically immensely fruitful (just think of the great concept of aromaticity) and aesthetically pleasing. Linguists could certainly do well with benzene: While my friends from the western part of Germany almost exclusively say benzene, my friends from the east speak of benzene, which, according to Iupac, is also the correct name.
And finally there is the old chemist's joke: "Does the theorist ask the organic scientist: 'What does oxygen actually do in cyclohexane?" "
Christian Remenyi, Frankfurt aM
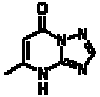
This triazolopyrimidine derivative is easily accessible from aminotriazole and acetoacetic ester, but is probably little known. In 1935 Emil Joachim Birr (in the first year of his work at the Wolfen film factory!) Discovered the excellent stabilizing effect of the substance on photographic silver halide emulsions and gave it the above code name. It remained a secret and a monopoly of Agfa until 1945. After that, it was included in almost every photo material worldwide. With the end of silver halide photography, the consolation remains that it now also plays a role in the synthesis of pharmaceuticals.
Gunther Fischer, Leipzig
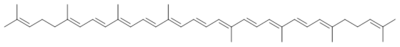
My favorite molecule is (all-E) -lycopene, an acyclic carotenoid that is responsible for the red color of tomatoes. This molecule has given me a lot of headache in recent years and has often been a challenge analytically. However, the molecule also contributed to establishing scientific and personal contacts, as I was able to coordinate an EU project for five years that dealt with the properties of this molecule. The molecule is also of culinary interest, as evidenced by the large number of recipes with tomatoes and tomato products.
Volker Böhm, Jena
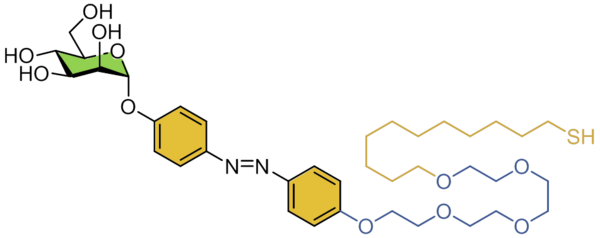
"The yellow of sugar": Sweet switches, like this azobenzene mannoside, have a yellow-orange color, form glyco-SAMs on gold surfaces and allow the reversible change of orientation of the sugar head group through irradiation: refined & controlled, useful & beautiful!
Thisbe K. Lindhorst, Kiel
By submitting their file, each participant declares that he / she agrees to the publication and the naming of his / her name. There is no legal entitlement to publication. By submitting their files, each participant declares that he / she is in possession of the image rights for images, graphics, etc. Graphics should depict the molecule almost correctly and can also be drawn by yourself. Participants can submit several favorite molecules, but each sender only takes part in the raffle once. If we include your molecule in our collection, we will inform you (not immediately, but via a collective confirmation every few weeks). Please understand that we cannot notify you if your molecule goes online. Just stop by here often.
This page has been machine translated. If you have any feedback or comments please feel free to contact us. 
last modified: 10.05.2021 15:09 H from K.J.Schmitz