

Xenon and the other noble gases have long been considered "unreactive". It was not until 1961 that the first compound of a noble gas was established. Although numerous xenon compounds are known today, many questions still remain unanswered and are the subject of current research. To the article about xenon and other noble gases on the website Faszination Chemie.
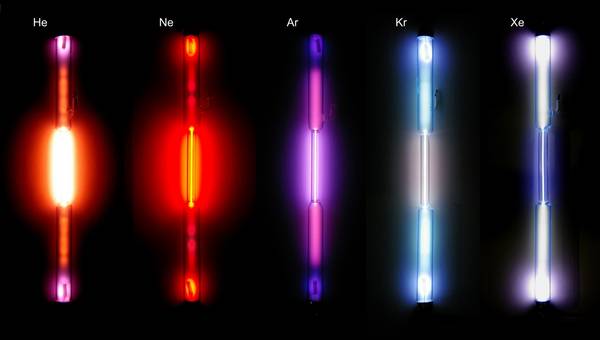
The noble gases form the 18th group and are on the far right in the periodic table. The noble gases include helium, neon, argon, krypton, xenon, radon and oganesson, the heaviest element known to date that can only be artificially produced for a very short time. Their electron configuration (filled shells) makes them very stable and therefore inert. The noble gases (except for Organesson) are described in this video from dahewissenChemie.
back to the periodic table
back to the start page for the year of the periodic table
to our brochure Chemistry of the Elements
published June 2019 with articles on 43 elements (7 MB, 160 pages)
Photo credits : Alchemist-hp www.pse-mendelejew.de,noble gases in discharge tubes,CC BY-SA 2.0 DE
This page has been machine translated. If you have any feedback or comments please feel free to contact us. 
last modified: 10.05.2021 16:39 H from K.J.Schmitz