

Here you will find articles that explain chemical phenomena or current research results in easy-to-understand language. This page was created during the first corona-related lockdown in spring 2020. The goal was to bring chemistry home to students, teachers, parents and anyone else who was interested.
A list with links to all contributions is compiled at the bottom of this page. All articles can be read free of charge without registration. The shortcut for this page is www.gdch.de/chemistryathome
Since the beginning of the corona pandemic, we have been washing our hands frequently and thoroughly. How does a side or a disinfectant actually work and what happens to the viruses or other unwanted substances on our skin? This is explained in our article on soap and our fact sheet Chemistry against viruses: hand washing or disinfection.
This marks the end of our Chemistry at Home campaign after three and a half months and more than 60 contributions. The long holidays have begun in several federal states and are giving schoolchildren, teachers and parents a breather. The number of corona infected people in Germany has fallen to a low level and hopefully after the summer holidays all pupils will be able to go to school every day again.
We are pleased that our campaign was well received and thank you for the positive feedback. If you liked our articles, then take a look at Fascination Chemistry, where we always explain exciting chemistry in a generally understandable way, especially in the sections Knowledge and Facts and Chemistry Everywhere. Stay healthy!

Crispr / Cas is a genetic engineering method that rewrites the DNA building blocks in the genome of a target organism and can thus specifically change the DNA. Proponents see genome editing as an opportunity for plant breeding to obtain new plant varieties that are adapted to climate change, among other things. Opponents warn of unexpected biological properties of the edited plants, arguing that the genetic engineering method circumvents natural rules such as inheritance. In this pros and cons, we explain with some vivid videos how Crispr Cas works and let a proponent and opponent each have their say.

Our youngest and even more so their parents benefit from chemistry. Because diapers keep much drier today than in earlier times. No rocket science, but exciting chemistry. Superabsorbents play an important role in this. In this article, you can find out what superabsorbents actually are and why they can absorb so much liquid.

Beta-carotene is the precursor of vitamin A, a vital vitamin that, among other things, maintains the function of our immune system and the visual process. It belongs to the group of substances called carotenoids, whose name comes from carrots, from which carotene was first isolated. But beta-carotene is not only a precursor to vitamin A, the food industry also uses it as a food coloring, for example as an addition to butter, confectionery or lemonades. Today's post explains how the pigment is converted into a vitamin and where beta-carotene still occurs in nature. https://faszinationchemie.de/chemie-ueberall/news/was-ist-eigentlich-beta-carotinnbsp/

We start the new week with a puzzle. Which element are we looking for? We will publish the solution at the bottom of this page in a few days.

Plastic has become an essential part of our everyday life. Our modern life can hardly be imagined without plastics. With their diverse properties, plastics have made numerous technical and medical innovations possible. But it not only brings blessings, it also presents us with a major challenge: plastic in the environment. Researchers have now been able to detect microplastics from the deep sea to the mountains. But how does the plastic get there? And is there a danger from the tiny particles? You will find out in today's post.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

In healthy people, the pancreas produces insulin - a hormone that regulates our blood sugar levels. Insulin transports the sugar that we ingest with food from the blood into the cells - these then burn the sugar for energy production or store it. But for some people the amount of insulin produced is insufficient or no insulin is produced at all. Such a lack of insulin leads to an increased blood sugar level, which in the long term can damage bodies . Some people have to put insulin into their bodies. "Diabetes" (diabetes mellitus) is a typical metabolic disease in today's affluent society. Today, insulin can be produced in large quantities using a biotechnological process. In the past, however, the hormone was isolated from the pancreas of animals. You can find out more about the discovery and production of the vital hormone in this article.
"Ask your doctor or pharmacist about risks and side effects" - we all know this saying. But before taking any medication, it is especially worthwhile to read the instructions for use on the package insert. Because there you will find important information on taking the drug and when, for example, it must not be used and which side effects have been observed in clinical studies. These life-saving cues are insights that are gained in the development of new drugs through two disciplines: pharmacokinetics and pharmacodynamics. The author of today's article explains the role these disciplines play in the development of new active ingredients.


As early as the early Bronze Age, lead was used to make various everyday objects - from vases to coffins. It was used in the manufacture of water pipes from the Roman Empire until the 1970s. But lead is dangerous to health; With chronic lead exposure, lead builds up in bones, teeth and hair and can lead to symptoms such as muscle weakness or fatigue. The Romans also added lead to wine in the form of lead acetate as a sweetener - it is believed that this led to chronic lead poisoning among citizens of higher rank and may have contributed to the fall of the Roman Empire. Today, 60 percent of the extracted lead goes into the production of lead accumulators, which are used as car batteries. All over the world, lead in the form of tetraethyl lead was added to petrol as an "anti-knock agent" because it reduced uncontrolled ignitions of the fuel mixture. The further development of engine technology made it possible that today - with a few exceptions - only unleaded petrol is sold worldwide. This article explains the role lead ions play in the next generation of solar cells.
Whether it's a fleece jacket, sportswear or the disposable beverage bottle - many items that we use in our everyday life contain polyester. Most of us are probably familiar with polyester: polyethylene terephthalate, better known as PET. We also encounter them in nature: Earth bees produce polyester and use it to line their nests. But what exactly is polyester and how is it made? You will find out in this article.

Around five to six liters flow permanently through our body: blood is the major transport medium in the human body. And it contains some information about our state of health. Infections, tumors or lipid metabolism disorders - analyzing our blood reveals many pathological changes in the body. In this article, the author explains why taking blood samples can save lives.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!
Today we have another puzzle for you. Which element are we looking for? We will publish the solution at the bottom of this page in a few days.

Gummy bears, jelly or aspic - many foods contain gelatine. You benefit from their ability to gel. The main component of gelatine is collagen, which is found in skin, rinds, bones or connective tissue, for example. In addition to food, it is also used in drug capsules, among other things. In today's post you will learn how gelation works and in which applications we can still find gelatine.

Whether hiking, cycling or taking a walk - functional clothing is particularly popular to protect yourself from wind, water and dirt. To keep us dry and clean, many textiles often contain so-called per- and polyfluorinated carboxylic acids. They are water and dirt repellent - and usually for several hours. But these substances are considered to be environmentally harmful. And they could also damage our health. This article explains why we can do without per- and polyfluorinated carboxylic acids for normal everyday use and should rather use alternative textiles.

Admittedly, you don't play with food. But after exactly 50 educationally valuable contributions, today we will take a look at what happens when you dip a commercial cheeseburger in concentrated hydrochloric acid. (Between us: nothing that we as chemists would not expect.) Professor Martyn Poliakoff explains why the fate of the unfortunate cheeseburger in the experiment corresponds to what happens to the meatballs in our stomachs. And with that we just got the curve to the pedagogical demands of this series.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

?Sticking together like pitch and brimstone? - one of the idioms into which one of the few elements that occurs in nature in its elementary form has crept. Sulfur has been known to mankind since ancient times. Some of its chemical compounds smell unpleasant - but environmental problems such as acid rain caused by the emission of sulfur dioxide have also contributed to sulfur's bad reputation. However, elemental sulfur itself is a yellow solid under standard conditions that is non-toxic and odorless. The chemical industry benefits from it as one of the most important raw materials. And the human organism cannot do without sulfur either. This article explains why "it stinks of sulfur" shouldn't be blamed on the element sulfur alone.

We Germans love chocolate: Germans ate around 11 kilograms per capita in 2017. But cocoa accumulates the toxic heavy metal cadmium. The darker the chocolate, the more cadmium. Since January 1, 2019, cocoa products may only contain a certain amount of the heavy metal. But how much cadmium does our chocolate bar contain? And how many bars of chocolate per week are harmful? The author takes us on the journey from the raw material to the finished chocolate bar.

Today we're going to look at the difference between the ball that was used as soccer in the past and the one that is used to play soccer today. Football used to be made of leather and soaked in water when it rained. If you got the ball on your head, it could hurt a lot - as heavy as it was through the water. The surface is now made of plastic, more precisely of polyurethane. In this article you can find out how football benefits from this material .
Chemistry is a difficult subject! Some of what you learned in school you forget over time. Unfortunately, this is also the case for some journalists. And in the stressful day-to-day editorial work, there is often no time to research the facts in more detail. It can happen, for example, that "chlorine gas" (German Chlorgas) becomes the new dangerous substance "Chorgas" (German "Chor" = Choir). Every year, there are some reports in the media that misrepresent chemical formulas or names. Much of it is very amusing for chemists. Some of these "fake news" from the past year are summarized here.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

Today there is another riddle. Which element are we looking for? We will publish the solution at the bottom of this page in a few days.

Today we deal with so-called low molecular weight active substances - molecules whose mass does not exceed 800 grams per mole. They have been produced and administered since the development of active substances began - and most of the drugs approved on the market today are mostly low-molecular active substances. Apart from their size, however, they don't have much in common: whether classic headache medication or modern cancer therapy, the small all-rounders cover almost every therapeutic area in Medicine . This article explains how these small molecules achieve great things.

Vanilla - the second most expensive spice worldwide and at the same time a popular flavor, for example for ice cream. The main component of the vanilla extract is vanillin. We find its pleasantly sweet note whether in pudding and cookies or in perfumes and cosmetics. In nature, vanillin occurs in the pods of spiced vanilla. Wine and whiskey that have matured in oak barrels also develop a flavor note that is created by the vanillin produced during storage. You can find out here how the vanillin is formed during storage.
Today we deal with humic substances. These are organic compounds that are found almost everywhere in our ecosystem - for example in the ground. They are responsible for maintaining the soil's microbiome. With their properties, however, they also play an important role in protecting the climate. Is it possible to save the climate with these substances? You will find out in this article.


Today we are dealing with the element oxygen - without it, life on earth would not be possible. Oxygen is the most common element on earth. One fifth of our air consists of oxygen, almost half of the earth's crust and 86% of the oceans. Oxygen is involved in combustion and corrosion processes and at the same time represents one of the most important industrial chemicals. Today's contribution explains what constitutes the element that we take in with every breath.
Oxygen reacts explosively with hydrogen (H 2 ) to form water; this is the famous oxyhydrogen reaction. We explain how this reaction works with the help of a few videos.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

In our everyday life we encounter it in glass, but also as a food additive: silicon dioxide. In our living space, silicon dioxide is one of the most common building blocks of inanimate (inorganic) matter. In nature, its most common form of appearance is quartz, which we find in various processed forms as a gem stone - for example the water-clear rock crystal or the purple amethyst. This article explains other manifestations and applications in our everyday life.
Have you already had a cup of coffee today? Today you will learn why you need more than just coffee beans for a fully automatic coffee machine and why you should descale it regularly despite the water filter. In this article, the author takes us into the inner workings of her fully automatic coffee machine.


How can we act in such a way that we conserve natural resources and at the same time secure our standard of living? Man-made climate change, declining biodiversity and plastic in the oceans pose great challenges for mankind. They require reflection and perhaps also a rethinking of how we want to live and do business in the future. The bioeconomy plays a decisive role in a possible change. What this term means and what role chemistry plays in it, you will find out in this article.
The first active ingredients against the virus that triggered the corona pandemic are currently being tested. And there is hope worldwide that an effective drug will be approved quickly. But how do you actually develop a new drug? And how does research work? The chemist and virologist Helga Rübsamen-Schaeff has specialized in the research and development of antiviral drugs against, among other things, herpes, cytomegalovirus and HIV. We asked them what to consider when developing a drug. Here she explains why setbacks are necessary to research in order to get ahead.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

Today we have something to guess. Which element are we looking for? We will publish the solution at the bottom of this page in a few days.


Today we are concerned with why species protection can protect not only animals, but also people. The current Covid-19 pandemic was triggered by a virus that jumped from animals to humans - such infectious diseases are called zoonoses. Epidemics such as the plague in the Middle Ages or the current Covid-19 pandemic have only occurred since thousands or millions of people have been living closely together. But how can we avoid future pandemics? The author of today's post explains this to us and shows what we can learn from the current pandemic.

The chemist Robert Wilhelm Bunsen and the physicist Gustav Robert Kirchhoff discovered the specific spectral lines of cesium in the dry residue of the Bad Dürkheim mineral water. This made cesium the first element to be identified using modern spectroscopic methods. Only the stable isotope 133 Cs occurs in nature. However, with the Chernobyl nuclear disaster in 1986, the element became known to large parts of the population in the form of the radioactive fission product 137 Cs when it was released into the environment in large quantities. At around 40 euros per gram, the price for cesium is currently around the same level as the gold price - which is why smuggling of the element is also taking place. In this article, the author takes us into the world of cesium, the base gold.

Gene therapy is a novel method of Medicine. This involves inserting genes into cells or tissue, for example to treat genetic defects. It already helps some patients in different areas of application, who without it would have no chance of therapy and thus often of survival. Oncology and the treatment of rare diseases are particularly benefiting from the progress made in gene-based therapies. On 6.5. we have stated that rare diseases are difficult to diagnose and research. Today's article looks at the hope that gene therapy offers for the treatment of rare diseases.
Whether fingernails, liver cells or the enzymes that control all functions and reactions in our body: They consist of proteins made up of amino acids. The amino acids are essential for us. However, our body cannot produce all of the amino acids itself. Therefore, we have to take in the amino acids that it cannot produce - so-called essential amino acids - with our food. But how does our body then convert the food so that it can get the amino acids it needs and use them for the intended functions? This is what this article explains.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!

Today we are talking about element 104 of the periodic table: rutherfordium. It does not occur naturally or in nuclear reactors. That is why it has to be generated artificially, i.e. in a nuclear reaction at an accelerator - also known as transmutation. In this article, the author takes us into the exciting world of nuclear reaction and explains how element 104 got its name.
Today we dedicate ourselves to diseases that have hardly been researched, little known and difficult to treat - but always complex, mostly undetected and often fatal: Rare diseases. A disease is considered rare in the EU if it affects no more than five in 10,000 people. This definition encompasses around 8,000 diseases, including rare forms of cancer, diseases of the cardiovascular system and autoimmune diseases. Around four million people in Germany suffer from a rare disease. In this article, you will find out why diagnosing and researching rare diseases is so difficult. Please note: After the introduction today, we will publish another article with interesting facts on the subject in the coming days.


So that we can grow, we need nitrogen compounds - just like all living things. Almost four fifths of the air we breathe consists of gaseous nitrogen, but only a few living things can use the nitrogen from the air. The nodule bacteria are one of the few living things that live in their roots from legumes ("pulses"). You can convert around 50 to 150 kilograms of nitrogen from the air into nitrogen compounds per hectare. If we eat meat or vegetables, we absorb the nitrogen compounds of the animals and plants. But how does nitrogen get into the plant if it is not a legume? This article explains this question to us today.
We start the new week with a puzzle. Which element are we looking for? We will deliver the solution in a few days at the bottom of this page.


For thousands of years, a person's hairstyle has been a means of expressing an individual personality, a social position or a political attitude. But hair contains much more information. Active ingredients that are absorbed into the body, consciously or unconsciously, healthy or harmful, can be detected in the hair. Forensic hair analyzes are carried out to check whether foreign substances such as poisons, drugs or medication are present. They serve, for example, as evidence in court proceedings. Today's article takes us into the exciting world of forensic hair analysis.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!
Salad sauces taste bland without acid. Wine becomes acidic if we let it stand - acetic acid is formed. Among other things, this is involved in the production of "aspirin". We also encounter acetic acid when cleaning. But what is acetic acid and how is vinegar made? We explain that today in this post.

In addition to the bonds between atoms and ions within a molecule, there are also interactions between molecules, so-called intermolecular interactions. However, these forces are weaker than the binding forces. The intermolecular interactions are partly responsible for the properties of a substance and influence, among other things, melting and boiling temperatures. These animations explain how the forces work. So that you can hear the explanations, you should turn on the sound.

Energy carrier, elixir of life, weapon and chemical reagent: hydrogen, number one in the periodic table and at the same time the star among the elements. Not only is the sun mainly made up of it, living organisms also contain hydrogen bound in some form; only carbon and oxygen play a greater role here. The sun's fuel is essential as a source of heat and light for life on earth.
The possibility of using hydrogen as a fuel was discovered as early as the 18th century. After the two world wars, the interest in hydrogen moved more and more into the focus of synthetic chemistry, among other things for the production of pharmaceuticals and pesticides. Nonetheless, the element also remained a topic for war weapons research. Modern energy research focuses on hydrogen and water. This article explains the role hydrogen plays in our universe and what we use it for today.

Whether in muesli or coffee, whether yoghurt or ice cream - we consume milk or dairy products every day. However, some people cannot tolerate milk, and most of them are lactose intolerant. In addition to normal dairy products, there are often lactose-free alternatives in supermarkets. But what exactly is lactose? And why do some of us not tolerate milk? We explain that to you here.
We say goodbye for this week and look forward to seeing you again next week. Stay healthy!
Today you can puzzle again. Which element are we looking for? We will deliver the solution in a few days at the bottom of this page.

In 399 BC The Athenian philosopher Socrates was executed by drinking the so-called hemlock cup, a drink made from the fruits and roots of the spotted hemlock. So-called alkaloids (?-conicein and coniin) were deadly. These hemlock poisons cause a gradual paralysis of the body until the poisoned suffocates - while fully conscious. Plants produce the poisonous substances to protect themselves from predators. Medicinal active ingredients are based on selected of these substances. In nature, they can be dangerous for humans and animals, including hemlock poisons. For this reason, agriculture is displacing the spotted hemlock and its poisons are not used medicinally today. This article explains how ?-conicein and coniin are chemically structured and why they are so toxic.

Today we deal with hormones, messenger substances in our body. They regulate and control many processes in our body, e.g. the metabolism - and are therefore indispensable. Various factors such as stress or pregnancy can affect hormone levels. But hormones also provide information about some diseases. A changed hormone concentration in body fluids can indicate a tumor or high blood pressure, for example. Today's post explains how hormones can be detected in our body.
Mercury was already used in ancient times. As the only metal that is liquid at room temperature, it is still fascinating today. In early times, mercury was a symbol of eternal life and was associated with gods such as Hermes, the messenger of the gods in Greek mythology. Mercury was used in Medicine, among other things. For example, intestinal obstructions used to be treated with metallic mercury. To this day it is used in pharmacology, even if its use has declined sharply and mercury is often replaced by similarly effective agents.
Because of its toxicity, mercury has now fallen into disrepute. Many of us may associate mercury with clinical thermometers, others may also know it from amalgam dental fillings. Nowadays, mercury is the epitome of poison, environmental degradation and industrial contamination of entire regions. This article explains how the image change came about.


Today we introduce a chemical substance that, with clever Marketing, gives cosmetics a touch of something special: urea, or better known as urea. Many of us have probably already come across this term under the ingredients of day or night creams. But what exactly is urea or urea? This is what this article explains - and shows that urea and urea are one and the same compound.
We say goodbye for this week and look forward to seeing you again next week. Stay healthy!
Ants of the species Megaponera analis hunt termites in large groups. If individual animals are injured on the hunt, they send out a chemical call for help, which calls a species-specific medical service to the scene. Today's post explains how this chemical call for help works.


Our brain consists of a huge network of around a hundred billion nerve cells, the neurons. These are connected to one another via contact points (synapses), exchange information and save it. In the brain of an Alzheimer's patient, these connections are cut. Depending on which area in our brain is affected, for example short-term memory or language skills are affected. As we already saw in the post from April 09 explained, the cause of the disease is unknown. However, research observes, for example, that certain proteins are deposited in the affected areas and form so-called "plaques". This article presents other current approaches in Alzheimer's research.
Cyclodextrins are ring-shaped molecules made up of glucose building blocks. They can trap lipophilic substances inside them, thereby changing their properties. The food industry uses its special structure, for example, as a whipping agent for desserts or for flavors. But how do the ring-shaped molecules come about? And how do they work? You can see this in a nice animation on this page from Wacker Chemie AG. So that you can hear the explanation, you should turn on the sound.
And you can do puzzles again today. Which element are we looking for? We will deliver the solution in a few days at the bottom of this page.


Alzheimer's disease is responsible for around 60-80 percent of the 24 million dementia cases worldwide and is characterized by increasing dementia. Factors that increase the risk of disease are known, but the cause is unknown. Existing drugs alleviate the symptoms or delay their occurrence - they cannot stop the disease. This article explains how Alzheimer's dementia makes diagnosis difficult. Please note: After the introduction today, we will publish another article with interesting facts on the subject in the coming days.
We say goodbye for this week and wish everyone happy Easter holidays. We look forward to seeing you again next week - it will continue on Tuesday (April 14th).

Hydrothermal springs on the ocean floor spew hot water that contains substances such as copper, iron and manganese salts. If they hit cold lake water, they pile up to form meter-high mountains of minerals. This article explains how these underwater sources are created.
Today we present you an experiment that creates a very voluminous foam, reminiscent of oversized toothpaste. The experiment is therefore also known as elephant toothpaste. Here we have put together some nice videos and explain the chemistry behind the experiment to you.

In countries without clean tap water, they are a vital source of drinking water. And with us, too, many appreciate them as a loyal companion in everyday life, during sports or on vacation: plastic beverage bottles. At the same time, the one-way plastic bottles are coming under increasing criticism due to the increasingly visible consequences for our environment. Today we explain why water in plastic (bottles) leads to (micro) plastic in water even when properly recycled. To the contribution.

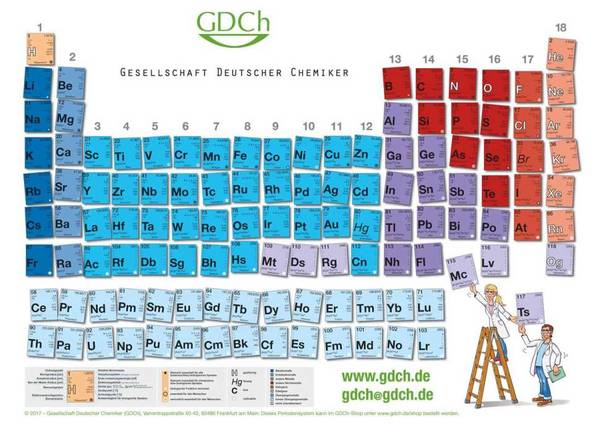
The periodic table is a fascinating system of order that nature has given the elements. The Russian chemist Dimitri Mendelejew and the German chemist Lothar Meyer recognized this almost simultaneously in 1869, independently of one another. For most chemists, the periodic table is set in stone - but occasionally people get creative and vary the usual order of the elements. We show you an overview of different variations of the periodic table here.
And you can do puzzles again today. Which element are we looking for? We will deliver the solution in a few days at the bottom of this page.
With that we say goodbye for this week and look forward to seeing you again next week. Stay healthy!
In 2009, around every fourth German between the ages of 25 and 34 had a tattoo. But what actually happens under the skin when and after the tattoo ink is applied? In this article we can trace a tattoo from a chemical point of view. There was an introduction to the topic last week. We explained what tattoo inks are made of (see below Chemistry at Home from 03/27/2020).

Whether on the roller coaster ride, cheering for the Bundesliga match in the championship or running a marathon - each and every one of us is sure to have had an ?adrenaline rush? at least once. These events may not be possible at the moment because you are at home - but today we will explain to you what happens in our body when there is an "adrenaline rush". And next time you stand in front of the roller coaster, you might think of us. To the contribution.

Our topic today is aimed at students * Secondary I. How is actually formed a polymer of the raw materials? And why can a product be solid even though its raw materials are liquid? And how do the individual components of an elastic polymer "bend" when you pull or push on it? You can see this in a few nice animations on this page from Wacker Chemie AG. So that you can hear the explanations, you should turn on the sound.
We are all familiar with the sight of a candle flame. Warm, light yellow, and shaped like a tear, it hugs the wick tightly. But what happens when you put a candle flame in weightlessness? We start the new week with this question. You can find the answer here.

Tattoos are in and have been for more than 5000 years. After all, the famous Ice Age man Ötzi had many different signs on his skin. What are tattoo inks actually made of? In this article we clarify that. Please note: After the introduction today, we will publish a second article in a few days with interesting facts about tattoos.
We say goodbye for this week and look forward to seeing you again next week. Stay healthy!
Today we have a riddle for you: which element are you looking for? We will deliver the solution in a few days at the bottom of this page.
Today we are devoting ourselves to a medical topic: In the case of headache or aching limbs, a pain pill often helps to relieve the pain. There are thousands of drugs used to treat a variety of diseases. But how is a drug made? To the contribution
Today we are dealing with the recycling of plastic waste: Chemical recycling converts the products into crude oil and thus apparently makes ideal use of the raw materials in plastic waste, but mechanical recycling is more environmentally friendly: cracking or melting? To the contribution
Today we are introducing a molecule that on the one hand protects us as a kind of protective layer in the stratosphere, on the other hand it damages us close to the ground: What actually is ozone, how is it created and how does it work? To the contribution
Welcome to Chemistry at Home. Today we are starting our series and are the first to introduce substances that everyone, whether they want to or not, has to do with: What are preservatives and how do they work in our food? To the contribution
Would you rather have something to guess? Then we have a riddle for you here. Which element are you looking for? We will deliver the solution in a few days at the bottom of this page.
On March 20th we asked about an element that is particularly important for counterfeit analysts, among other things. The solution is chrome.
On March 26th we asked about an element which, among other things, leads to "intoxicating" states in many people. The solution is gold.
On April 3rd we asked about an element that occurs in the fuel of solid rocket rockets, but also serves as a food additive. The solution is aluminum.
On April 14th we asked about an element that only ever existed in two places in the world. The solution is Tenness.
On April 23 we asked about an element that can improve glucose tolerance in type 2 diabetics. The solution is cobalt.
On May 4th we asked about an element whose isotope, which he discovered first, has a half-life of only 1.17 minutes. The solution is Protactinium.
On May 14th we asked about an element that often makes a loud appearance with one of its most famous reactions at chemical show lectures. The solution is hydrogen.
On May 28 we asked about an element whose main isotope has a half-life roughly equivalent to the age of the earth. The answer is uranium.
On June 10th we asked about an element whose discoverer did his doctorate on vegetable oils. The solution is tantalum.
On June 22nd we asked about an element whose modifications are popular with draftsmen, architects and girls. The solution is carbon.
The most recent posts can be found above. To view older posts, click the green arrow next to the heading.
GDCh public relations
pr@gdch.de
This page has been machine translated. If you have any feedback or comments please feel free to contact us. 
last modified: 11.11.2021 14:02 H from K.J.Schmitz